Notre expertise

Vapeur

Oxyde d’éthylène
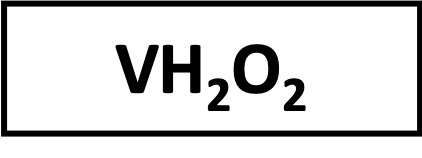
Péroxide
Les aspects suivants doivent être pris en compte lors d’un audit du système qualité:
- Relations avec les services internes (achats, hygiène, biomédicale, technique, transport, bloc opératoires, …)
- Exigences réglementaires (exigences spécifiques nationales, normes et directives applicables, …)
- Infrastructure (maintenance, qualification, suivi, consommables, …)
- Maîtrise des processus (nettoyage, désinfection, conditionnement, stérilisation, stockage)
- Contrôle de processus (contrôles périodiques en fonction de la gestion des risques, utilisation d’indicateurs, contrôle des paramètres, …)
- Application des règles de classification (RKI, Spaulding, …)
- Validation des processus (nettoyage-désinfection, stérilisation, emballage)
- Traçabilité (libération de charges, documentation des charges, validation logicielle)


